Abstract
Introduction: Pegfilgrastim, a long-acting granulocyte colony-stimulating factor (G-CSF), is indicated for reduction of incidence of febrile neutropenia in patients with non-myeloid malignancies receiving myelosuppressive chemotherapy. Here, the immunogenicity data for MYL-1401H, a proposed biosimilar of pegfilgrastim, from the clinical studies will be presented.
Methods: The efficacy, safety, and immunogenicity of MYL-1401H were investigated in 3 clinical studies, 2 in healthy volunteers (HV) and 1 in patients receiving chemotherapy. MYL-1401H-1001 was a 3-way crossover study comparing the pharmacokinetics (PK) and pharmacodynamics (PD) of a single 2-mg dose of MYL-1401H with reference pegfilgrastim (Neulasta®) sourced from both the European Union (EU) and United States (US) in HV. MYL-1401H-1002 was a parallel-group study designed to compare the immunogenicity of MYL-1401H with US-pegfilgrastim at a repeated 6-mg dose in HV. MYL-1401H-3001 was a multicenter, randomized, double-blind, parallel-group equivalence study of MYL-1401H 6 mg vs EU-pegfilgrastim 6 mg in patients with breast cancer receiving chemotherapy for 6 cycles. The primary efficacy endpoint in MYL-1401H-3001 was duration of severe neutropenia in cycle 1, defined as days with absolute neutrophil count <0.5 × 109/L. A thorough assessment of immunogenicity was conducted across the 3 studies. The antidrug antibody (ADA; Covance, Chantilly, VA) analysis was performed using a validated, sensitive, and specific analytical method on the MesoScale Discovery® Platform (Rockville, MD). Serum samples were analyzed for the presence of ADA against MYL-1401H, EU-pegfilgrastim, and US-pegfilgrastim. Samples that resulted positive in the screening assay were further evaluated in a confirmatory assay. The samples confirmed as ADA positive were titrated to elucidate relative levels of the ADA response and further evaluated for domain characterization to determine if the antibodies were specifically directed against the polyethylene glycol (PEG) or the G-CSF domain of the molecule, or both. Samples confirmed as positive for ADA were analyzed for neutralizing antibodies (NAb; CIRION BioPharma Research) using a validated cell-based assay.
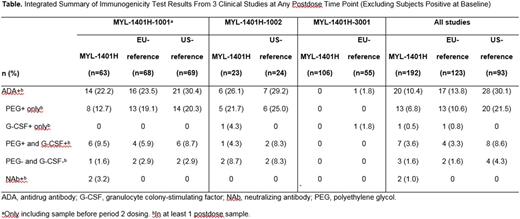
Results: Overall, these studies demonstrated that the PK, PD, efficacy, and safety of MYL-1401H were equivalent to reference pegfilgrastim. Across all 3 studies, the proportions of subjects who were ADA positive before dosing were similar in the MYL-1401H (30/223; 13.5%) and EU-pegfilgrastim groups (16/139; 11.5%) and were lower in the US-pegfilgrastim group (4/97; 4.1%). Most (86.0%) of the subjects with ADA had antibodies that recognized the PEG domain, which may be attributable to exposure to PEG-related compounds in the environment as well as the highly sensitive nature of the assay, which can detect very low levels of antibodies. Treatment-induced impact on immunogenicity across the 3 studies was evaluated by analyzing the postdose ADA results excluding those subjects who were ADA positive at baseline (Table). Overall, the proportions of subjects with postdose treatment-induced ADA were similar in the MYL-1401H (20/192; 10.4%) and EU-pegfilgrastim groups (17/123; 13.8%), while they were higher in the US-pegfilgrastim group (28/93; 30.1%). Although the proportion of subjects with treatment-induced ADA was higher in the US-pegfilgrastim group, in most cases, the ADA recognized only the PEG moiety, the titers were low, and the antibodies were non-neutralizing. Two subjects (1 each in the MYL-1401H and EU-pegfilgrastim groups) had antibodies against the G-CSF moiety only. Two subjects were NAb positive in the MYL-1401H group, while none were NAb positive in either reference pegfilgrastim group. There was no evidence of loss of efficacy or severe treatment-induced immune-related adverse events in ADA- or NAb-positive subjects.
Conclusion: Results in HV indicate that MYL-1401H does not induce either early or late immune response and has a low immunogenic potential, similar to that of EU- and US-pegfilgrastim, after repeated administration in a sensitive population without any confounding illness or immunosuppressive medications. Similarly, the immunogenic potentials of MYL-1401H and EU-pegfilgrastim are low in patients with breast cancer. Overall, MYL-1401H has an immunogenic profile similar to that of EU-pegfilgrastim and US-pegfilgrastim.
Waller: Mylan: Consultancy, Honoraria. Ranganna: Mylan Pharmaceuticals Inc: Employment. Pennella: Mylan: Employment. Mattano: HARP Pharma Consulting, LLC: Employment. Loa: Mylan Pharmaceuticals Inc: Employment. Donnelly: Mylan Pharmaceuticals Inc: Employment. Liu: Mylan Pharmaceuticals Inc: Employment. Watson: Covance: Employment. St-Jean: CIRION BioPharma Research Inc: Employment. Chatterjee: Biocon Research Ltd: Employment. Nayak: Biocon Research Ltd: Employment. Sengupta: Biocon Research Ltd: Employment. Kothekar: Biocon Research Ltd: Employment. Barve: Mylan Pharmaceuticals Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.